GenoCellEdit
GENOME EDITING PLATFORM WITH CRISPR-Cas
The GenoCellEdit platform (PF-GCE) is associated to team 2 of the UMR INSERM 1064-CRTI. PF-GCE promotes genome editing technology, in particular through the CRISPR/Cas system. Since June 2014, it has been open to academic researchers from INSERM 1064-CRTI. Its R&D activities support innovative projects aimed at advancing technology and its applications. GCE is under the direction of Ignacio Anegon and Jean-Marie Heslan (IR) is the Technical Authority.
Technology
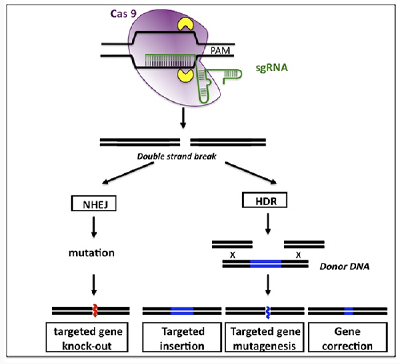 Artificials nucleases consist primarily of ZFNs, Meganucleases, Tales Nucleases and CRISPR/Cas. These three types of nucleases recognize specific sequences and generate DNA breaks through different mechanisms, but DNA repair pathways are common: NHEJ repair (non-homologous end joining) or homologous recombination in the presence of donor DNA. The NHEJ is a mechanism conducive to errors during repair which generates deletions and/or nucleotide insertions that can produce reading frame shifts causing gene invalidation (knock-out or KO). Homologous recombination allows new sequences to be introduced into the target gene (knock-in or KI). Thus, these artificial nucleases allow to generate both KO and/or KI.
Artificials nucleases consist primarily of ZFNs, Meganucleases, Tales Nucleases and CRISPR/Cas. These three types of nucleases recognize specific sequences and generate DNA breaks through different mechanisms, but DNA repair pathways are common: NHEJ repair (non-homologous end joining) or homologous recombination in the presence of donor DNA. The NHEJ is a mechanism conducive to errors during repair which generates deletions and/or nucleotide insertions that can produce reading frame shifts causing gene invalidation (knock-out or KO). Homologous recombination allows new sequences to be introduced into the target gene (knock-in or KI). Thus, these artificial nucleases allow to generate both KO and/or KI.
Some applications
- Gene Invalidation (Knock-out)
- Insertion of exogenous DNA into a specific genomic locus (KI)
- Insertion of a specific mutation
- Genome modification of different species (e.g., human cells, mice, rats, etc.)
- Gene marking (KI of a fluorescent reporter gene)
- Genome editing in IPS cells
- Resistance to infectious agents
- Gene therapy: targeted integration, gene repair
Know-how
- In silico design of guide RNA targeting the genomic site of interest
- In cellulo functional validation of guide RNA
- DNA donor design to modify the genome and its detection
- Tool vectorization (plasmids, RNA, viral vectors)
- Production of mRNA
- Advice on strategy, type of Cas9, vectors and their uses
Type of services
- Basic service: design and validation of guide RNA, vectorization in a plasmid, advice and project support
- Analyses of off-target activity (depending on the species)
- DNA donor design and construction
- Vectorization as synthetic RNAs or in an all-in-1 recombinant viral vector (adenoviral vectors, lentiviral vectors)
- Technological training in the detection of induced mutations
- Custom service: depending on the user’s wishes, tasks can be shared with the platform for optimal use of the respective resources
- Research and development
Publications
- A variant of ASIC2 mediates sodium retention in nephrotic syndrome. JCI Insight. 9;6(15):e148588. https://doi-org.proxy.insermbiblio.inist.fr/10.1172/jci.insight.148588
- Advances in Genome Editing and Application to the Generation of Genetically Modified Rat Models. Front Genet. 12:615491. https://doi.org/10.3389/fgene.2021.615491
- Cardiovascular phenotype of the Dmdmdx rat - a suitable animal model for Duchenne muscular dystrophy. Dis Model Mech. 14(2):dmm047704. https://doi.org/10.1242/dmm.047704
- A rat model expressing a human amyloidogenic kappa light chain. Amyloid. https://doi-org.proxy.insermbiblio.inist.fr/10.1080/13506129.2021.1877651
- Overexpression of endothelial β3 -adrenergic receptor induces diastolic dysfunction in rats. ESC Heart Fail. https://doi-org.proxy.insermbiblio.inist.fr/10.1002/ehf2.13040
- Human MuStem Cell Grafting into Infarcted Rat Heart Attenuates Adverse Tissue Remodeling and Preserves Cardiac Function. Mol Ther Methods Clin Dev.https://doi-org.proxy.insermbiblio.inist.fr/10.1016/j.omtm.2020.06.009
- Otoferlin gene editing in sheep via CRISPR-assisted ssODN-mediated Homology Directed Repair. Scientific Reports, 10(1), 5995. https://doi.org/10.1038/s41598-020-62879-y
- In Vivo Analysis of Human Immune Responses in Immunodeficient Rats. Transplantation, 104(4), 715–723. https://doi.org/10.1097/TP.0000000000003047
- Comparison of Human and Experimental Pulmonary Veno-Occlusive Disease. American Journal of Respiratory Cell and Molecular Biology. https://doi.org/10.1165/rcmb.2019-0015OC
- Characterization of brain dystrophins absence and impact in dystrophin-deficient Dmdmdx rat model. PLoS ONE, 15(3). https://doi.org/10.1371/journal.pone.0230083
- Humanization of Immunodeficient Animals for the Modeling of Transplantation, Graft Versus Host Disease and Regenerative Medicine. Transplantation. https://doi.org/10.1097/TP.0000000000003177
- Regenerative cell therapy for the treatment of hyperbilirubinemic Gunn rats with fresh and frozen human induced pluripotent stem cells-derived hepatic stem cells. Xenotransplantation, 27(1), e12544. https://doi.org/10.1111/xen.12544
- Ceruloplasmin Deficiency Does Not Induce Macrophagic Iron Overload: Lessons from a New Rat Model of Hereditary Aceruloplasminemia. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, septembre, fj201901106R. https://doi.org/10.1096/fj.201901106R
- Characterization of Kcnk3-Mutated Rat, a Novel Model of Pulmonary Hypertension. Circulation Research 125 (7): 678‑95. https://doi.org/10.1161/CIRCRESAHA.119.314793
- Immunophenotype of a Rat Model of Duchenne’s Disease and Demonstration of Improved Muscle Strength After Anti-CD45RC Antibody Treatment. Frontiers in Immunology 10: 2131. https://doi.org/10.3389/fimmu.2019.02131
- Multispecific Antibody Development Platform Based on Human Heavy Chain Antibodies. Frontiers in Immunology 9: 3037. https://doi.org/10.3389/fimmu.2018.03037
- CtIP fusion to Cas9 enhances transgene integration by homology-dependent repair. Nature Communications, 9(1), 1133. https://doi.org/10.1038/s41467-018-03475-7
- Generation of Immunodeficient Rats With Rag1 and Il2rg Gene Deletions and Human Tissue Grafting Models. Transplantation, 102(8), 1271‑1278. https://doi.org/10.1097/TP.0000000000002251
- Breakdown of Immune Tolerance in AIRE-Deficient Rats Induces a Severe Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy-like Autoimmune Disease. Journal of Immunology (Baltimore, Md.: 1950), 201(3), 874‑887. https://doi.org/10.4049/jimmunol.1701318
- Immunological characterization of a rat model of Duchenne’s disease and demonstration of improved muscle strength after anti-CD45RC antibody treatment. BioRxiv, 407023. https://doi.org/10.1101/407023
- Cell-surface C-type lectin-like receptor CLEC-1 dampens dendritic cell activation and downstream Th17 responses. Blood Advances, 1(9), 557‑568. https://doi.org/10.1182/bloodadvances.2016002360
- Advances in transgenic animal models and techniques. Transgenic Research, 26(5), 703‑708. https://doi.org/10.1007/s11248-017-0038-x
- Antigen-specific single B cell sorting and expression-cloning from immunoglobulin humanized rats: a rapid and versatile method for the generation of high affinity and discriminative human monoclonal antibodies. BMC Biotechnology, 17(1), 3. https://doi.org/10.1186/s12896-016-0322-5
- Generation of gene-edited rats by delivery of CRISPR/Cas9 protein and donor DNA into intact zygotes using electroporation. Scientific Reports, 7(1), 16554. https://doi.org/10.1038/s41598-017-16328-y
- A Rapid and Cost-Effective Method for Genotyping Genome-Edited Animals: A Heteroduplex Mobility Assay Using Microfluidic Capillary Electrophoresis. Journal of Genetics and Genomics = Yi Chuan Xue Bao, 43(5), 341‑348. https://doi.org/10.1016/j.jgg.2016.04.005
- Pronuclear injection for the production of transgenic rats. Cold Spring Harbor protocols.
- Comparative Analysis of piggyBac, CRISPR/Cas9 and TALEN Mediated BAC Transgenesis in the Zygote for the Generation of Humanized SIRPA Rats. Scientific Reports, 6, 31455. https://doi.org/10.1038/srep31455
- IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunology, 9(2), 539‑549. https://doi.org/10.1038/mi.2015.83
- New insights and current tools for genetically engineered (GE) sheep and goats. Theriogenology, 86(1), 160‑169. https://doi.org/10.1016/j.theriogenology.2016.04.028
- Improved Genome Editing Efficiency and Flexibility Using Modified Oligonucleotides with TALEN and CRISPR-Cas9 Nucleases. Cell Reports, 14(9), 2263‑2272. https://doi.org/10.1016/j.celrep.2016.02.018
- Genome Editing in Rats Using TALE Nucleases. Methods in Molecular Biology (Clifton, N.J.), 1338, 245‑259. https://doi.org/10.1007/978-1-4939-2932-0_18
- Efficient Generation of Myostatin Knock-Out Sheep Using CRISPR/Cas9 Technology and Microinjection into Zygotes. PloS One, 10(8), e0136690. https://doi.org/10.1371/journal.pone.0136690
- Regulatory B Cells with a Partial Defect in CD40 Signaling and Overexpressing Granzyme B Transfer Allograft Tolerance in Rodents. Journal of Immunology (Baltimore, Md.: 1950), 195(10), 5035‑5044. https://doi.org/10.4049/jimmunol.1500429
- Homology-directed repair in rodent zygotes using Cas9 and TALEN engineered proteins. Scientific Reports, 5, 14410. https://doi.org/10.1038/srep14410
- Transgenic animals and genetic engineering techniques. Nantes, France, 2-3 July, 2015. Transgenic Research, 24(6), 1079‑1085. https://doi.org/10.1007/s11248-015-9904-6
- Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation, 131(11), 1006‑1018. https://doi.org/10.1161/CIRCULATIONAHA.114.008750
- Transgenic Animals Derived by DNA Microinjection. In S. Dübel & J. M. Reichert (Éd.), Handbook of Therapeutic Antibodies (p. 77‑88). Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. https://doi.org/10.1002/9783527682423.ch4
- Characterization of dystrophin deficient rats: a new model for Duchenne muscular dystrophy. PloS One, 9(10), e110371. https://doi.org/10.1371/journal.pone.0110371
- Gene targeting in rats using transcription activator-like effector nucleases. Methods (San Diego, Calif.), 69(1), 102‑107. https://doi.org/10.1016/j.ymeth.2014.02.027
- Generation of TALEN-mediated GRdim knock-in rats by homologous recombination. PloS One, 9(2), e88146. https://doi.org/10.1371/journal.pone.0088146
- Efficient gene targeting by homology-directed repair in rat zygotes using TALE nucleases. Genome Research, 24(8), 1371‑1383. https://doi.org/10.1101/gr.171538.113
- Human antibody expression in transgenic rats: comparison of chimeric IgH loci with human VH, D and JH but bearing different rat C-gene regions. Journal of Immunological Methods, 400‑401, 78‑86. https://doi.org/10.1016/j.jim.2013.10.007
- Generation of Rag1-knockout immunodeficient rats and mice using engineered meganucleases. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 27(2), 703‑711. https://doi.org/10.1096/fj.12-219907
- Technical advances in the generation of transgenic animals and in their applications. Nantes, France, June 7th 2013. Transgenic Research, 22(5), 1065‑1072. https://doi.org/10.1007/s11248-013-9736-1
- High-affinity IgG antibodies develop naturally in Ig-knockout rats carrying germline human IgH/Igκ/Igλ loci bearing the rat CH region. Journal of Immunology (Baltimore, Md.: 1950), 190(4), 1481‑1490. https://doi.org/10.4049/jimmunol.1203041
- Transgenesis and genome analysis, Nantes, France, June 6th 2011. Transgenic Research, 21(2), 449‑456. https://doi.org/10.1007/s11248-011-9541-7
- Effects of BCL-2 over-expression on B cells in transgenic rats and rat hybridomas. International Immunology, 23(10), 625‑636. https://doi.org/10.1093/intimm/dxr071
- Generation of Transgenic Rats Using Microinjection of Plasmid DNA or Lentiviral Vectors. In S. Pease & T. L. Saunders (Éd.), Advanced Protocols for Animal Transgenesis: An ISTT Manual (p. 117‑135). Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-20792-1_7
- Knockout rats generated by embryo microinjection of TALENs. Nature Biotechnology, 29(8), 695‑696. https://doi.org/10.1038/nbt.1940
- Generation of gene-specific mutated rats using zinc-finger nucleases. Methods in Molecular Biology (Clifton, N.J.), 597, 211‑225. https://doi.org/10.1007/978-1-60327-389-3_15
- Characterization of immunoglobulin heavy chain knockout rats. European Journal of Immunology, 40(10), 2932‑2941. https://doi.org/10.1002/eji.201040939
- Generation of transgenic rats by microinjection of short DNA fragments. Methods in Molecular Biology (Clifton, N.J.), 597, 81‑92. https://doi.org/10.1007/978-1-60327-389-3_6
- “Transgenesis, recent technical developments and applications” Nantes, 8th June 2009. Transgenic Research, 19(4), 711‑714. https://doi.org/10.1007/s11248-009-9340-6
- ). Zinc-finger nucleases: a powerful tool for genetic engineering of animals. Transgenic Research, 19(3), 363‑371. https://doi.org/10.1007/s11248-009-9323-7
- New lines of GFP transgenic rats relevant for regenerative medicine and gene therapy. Transgenic Research, 19(5), 745‑763. https://doi.org/10.1007/s11248-009-9352-2
- The use of lentiviral vectors to obtain transgenic rats. Methods in Molecular Biology (Clifton, N.J.), 597, 109‑125. https://doi.org/10.1007/978-1-60327-389-3_8
- Analysis by quantitative PCR of zygosity in genetically modified organisms. Methods in Molecular Biology (Clifton, N.J.), 597, 277‑285. https://doi.org/10.1007/978-1-60327-389-3_19
- Pronuclear DNA injection for the production of transgenic rats. Methods in Molecular Biology (Clifton, N.J.), 561, 73‑88. https://doi.org/10.1007/978-1-60327-019-9_5
- Knockout rats via embryo microinjection of zinc-finger nucleases. Science (New York, N.Y.), 325(5939), 433. https://doi.org/10.1126/science.1172447
- Study of the microcirculation in hDAF transgenic rat livers xenoperfused with human blood. Xenotransplantation, 16(2), 83‑90. https://doi.org/10.1111/j.1399-3089.2009.00519.x
- Transgenic Modifications of the Rat Genome. Transgenic Research, 14(5), 531–546. https://doi.org/10.1007/s11248-005-5077-z
- Transgenic expression of CTLA4-Ig by fetal pig neurons for xenotransplantation. Transgenic Research, 14(4), 373–384.
- Vascular beta-adrenergic remodeling in rat transgenic model over-expressing endothelial beta3-adrenoceptors. Archives Des Maladies Du Coeur Et Des Vaisseaux, 98(7–8), 836–840.
- Generation of heme oxygenase-1-transgenic rats. Experimental Biology and Medicine (Maywood, N.J.), 228(5), 466–471.
- No functional benefit for hDAF-transgenic rat livers despite protection from tissue damage following perfusion with human serum. Transplant International: Official Journal of the European Society for Organ Transplantation, 15(12), 595–601. https://doi.org/10.1007/s00147-002-0463-4
- Differential sensitivity of endothelial cells of various species to apoptosis induced by gene transfer of Fas ligand: Role of FLIP levels. Molecular Medicine (Cambridge, Mass.), 8(10), 612–623.
- lacZ transgenic rats tolerant for beta-galactosidase: Recipients for gene transfer studies using lacZ as a reporter gene. Human Gene Therapy, 13(11), 1383–1390. https://doi.org/10.1089/104303402760128603
- Cytotoxic immune response blunts long-term transgene expression after efficient retroviral-mediated hepatic gene transfer in rat. Molecular Therapy: The Journal of the American Society of Gene Therapy, 5(4), 388–396. https://doi.org/10.1006/mthe.2002.0561
- Rapid and accurate determination of zygosity in transgenic animals by real-time quantitative PCR. Transgenic Research, 11(1), 43–48.
- Cellular immunity overrules the protective effect of human DAF as demonstrated in an ex vivo heart perfusion model. Transplantation Proceedings, 33(1–2), 781–782.
- Cryopreservation procedure for 1-cell and two-cell stage transgenic rat embryos. Transgenics, 3, 237–242.
- Protection against hyperacute xenograft rejection of transgenic rat hearts expressing human decay accelerating factor (DAF) transplanted into primates. Molecular Medicine (Cambridge, Mass.), 5(9), 617–630.
- Optimization of cryopreservation procedures for rat embryos. Transplantation Proceedings, 31(3), 1531–1532.
- Endothelial expression of Fas ligand in transgenic rats under the temporal control of a tetracycline-inducible system. Transplantation Proceedings, 31(3), 1533–1534.
- Production of transgenic rats for human regulators of complement activation. Transplantation Proceedings, 29(3), 1770.
- Generation and use of transgenic rats. Rat Genome, 3, 125–132.
- of human CD59 tissue expression directed by the CMV-IE-1 promoter in transgenic rats. Transgenic Research, 5(6), 443–450.
- Transgenesis in rats: Technical aspects and models. Transgenic Research, 5(4), 223–234.
Contact information
Updated on 21 January 2022.