Team 4 : Deciphering organ immune regulation in inflammation and transplantation (DORI-t)
Team 4 leaders: Dr. Sophie Brouard & Pr. Magali Giral
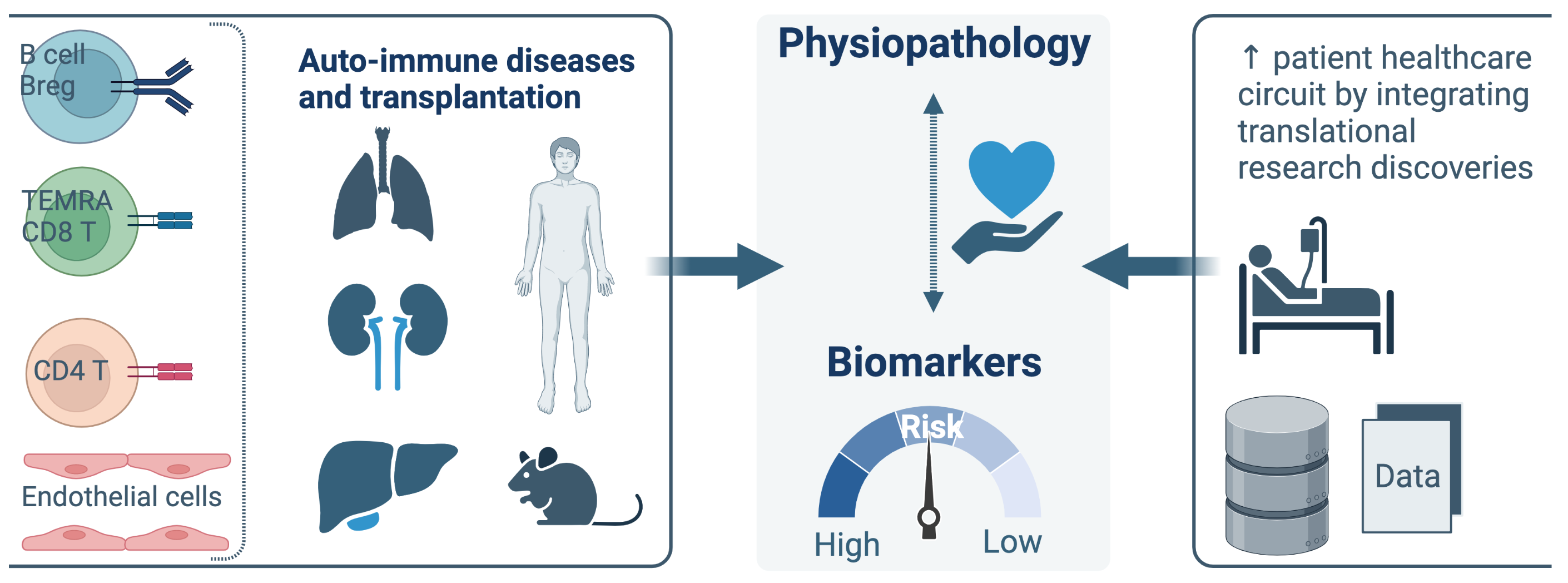
Team 4 has a long-standing expertise and interest in the analysis of immune responses in transplantation and autoimmune diseases. The contribution of adaptive cellular and humoral subsets is analyzed in the pathogenic response leading to allograft rejection and autoimmune diseases but also in the achievement of immune tolerance. Our research is focused primarily on human thanks to the tight connection with clinical departments, the establishment of large biocollection of human samples (Kidney and Lung allo-transplantation; Auto-immune hepatitis, Anca-associated vasculitis) and the large scientific expertise of Team4 researchers (B cell, CD4 and CD8 T cells, endothelial cells). DORI-t aims to integrate the knowledge from basic research studies into translational research programs to develop innovative healthcare strategies in solid organ transplantation (kidney, lung, liver) and in autoimmune diseases.

Research program
Team 4 has a long-standing expertise and interest in the analysis of immune responses in transplantation and autoimmune diseases. The contribution of adaptive cellular and humoral subsets is analyzed in the pathogenic response leading to allograft rejection and autoimmune diseases but also in the achievement of immune tolerance. Our research is focused primarily on human thanks to the tight connection with clinical departments, the establishment of large biocollection of human samples (Kidney and Lung allo-transplantation; Auto-immune hepatitis, Anca-associated vasculitis) and the large scientific expertise of Team4 researchers (B cell, CD4 and CD8 T cells, endothelial cells). DORI-t aims to integrate the knowledge from basic research studies into translational research programs to develop innovative healthcare strategies in solid organ transplantation (kidney, lung, liver) and in autoimmune diseases.
Basic research axis
We aim to decipher the dynamic equilibrium between effector and regulatory subsets of T cells (CD8, CD4), and B cells, endothelial cells (ECs) and their interactions in allo- and auto-immunity.
- Deciphering the mechanisms and specificity of Bregs. PI: S. Brouard, H. Le Mai. Characterize and decipher the mechanisms of Breg in transplantation and AID to translate GZMB+ Breg into clinical cellular therapy testing different approaches: GZMB+ Bregs (polyconal and Ag specific) and extracellular vesicles secreted by Bregs.
- Antigen-specific CD4 T cells in AutoImmune Liver Diseases (AILD). PI: S. Conchon, A. Renand. Determine if the CD4 T cell phenotype identified is disease-specific or if it is shared among hepatic autoimmune disorders, identify dominant epitopes from liver self-antigens to target autoreactive T cells, for the development of innovative specific therapeutic strategies, develop a blood immune biomarker based and to investigate the role of antigen-specific CD4 T cells in the transition between the natural tolerant liver environment and autoimmunity, with an original murine model.
- CD8 Treg in allogeneic transplantation. PI: S. Conchon, S. Brouard. Identify and characterize the human counterpart of the murine CD8 Treg population that we have identified in the liver and that exhibits allogeneic tolerance properties.
- Incidence of non-canonical, HLA-E-restricted, anti-HCMV CD8 T cell responses graft outcome. PI: B. Charreau. Decipher both the clinical input and the biology of the HLA-EUL40 CD8 T cells, an anti-HCMV CD8 T cell subset present in at least 30% of HCMV+ transplant recipients and represent up to >30% of total CD8 T cells post-infection.
- TEMRA CD8 in Kidney Transplantation. PI: Nicolas Degauque. Obtain a comprehensive mapping of the immune social network in kidney biopsies from Kidney Transplant Recipients and patients with Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), define the regulation of pathogenic TEMRA (Effector Memory re-expressing CD45RA) CD8 by effector and regulatory B cells, and define the regulation of pathogenic TEMRA CD8 by neutrophils.
Translational research axis
To enter the translational prognostic tools we’ve built for many years, in clinical and routine practices: PI: M. Giral, S. Brouard.
- Over the last decades and using large multicentric biocollection and using large multi centric bio collection and database DIVAT, we have identified biomarkers of high and low risk to graft failure. We now aim to implement these biomarkers in clinical and routine practices. The European AGORA project aims to cluster patients according to their chance of long-term graft survival using a stepwise algorithm based on minimally invasive blood biomakers and Kidney Transplant Failure Score (KTFS) and to perform a multicenter randomized open label trial of immunosuppression minimization (extra‐low Tacrolimus (TAC) vs standard of care) with stringent monitoring of TAC pharmacokinetics using finger‐prick test.
- Validate the feasibility and diagnostic accuracy of the Integrative Data System that we obtained in the first ongoing step of the KTD-Innov (RHU 2018-2023) project aiming to deliver precision diagnosis of kidney histology within the first year of transplantation. The validation of the product will be performed in a prospective open clinical study including 300 patients. This project is linked to the European H2020 EUTRAIN (PI A. Loupy).
- Continue the development of improving the predictive abilities of the dynamic KTFS (DynPG) and study the contribution of other longitudinal variables and particular attention to non-adherence to immunosuppression treatment.
Clinicians
Jérôme GOURNAY - PH
Aurélie MEURETTE - PH
Jean-François MOSNIER - PU-PH
Karine RENAUDIN - MCU-PH
Adrien TISSOT - PH
Aurélie MEURETTE - PH
Jean-François MOSNIER - PU-PH
Karine RENAUDIN - MCU-PH
Adrien TISSOT - PH
Research assistants
Eugénie DURAND - TH
Pierre-Jean GAVLOVSKY - IR
Nathalie GERARD - AI
Jean-Paul JUDOR - AI
Clarisse KERLEAU - IH
Vladimir KRASIKOV - IR
David LAIR - IHP
Marine LORENT - IR
Elisabeth NGUYEN - IR
Emmanuelle PAPUCHON - IH
Gaëlle TILLY - IE
Pierre-Jean GAVLOVSKY - IR
Nathalie GERARD - AI
Jean-Paul JUDOR - AI
Clarisse KERLEAU - IH
Vladimir KRASIKOV - IR
David LAIR - IHP
Marine LORENT - IR
Elisabeth NGUYEN - IR
Emmanuelle PAPUCHON - IH
Gaëlle TILLY - IE
Associate Researchers
Postdoctoral fellows
Selected publications

Single cell profiling of circulating autoreactive CD4 T cells from patients with autoimmune liver diseases suggests tissue imprinting. Cardon A, Guinebretière T, Dong C, Gil L, Ado S, Gavlovsky PJ, Braud M, Danger R, Schultheiß C, Doméné A, Paul-Gilloteaux P, Chevalier C, Bernier L, Judor JP, Fourgeux C, Imbert A, Khaldi M, Bardou-Jacquet E, Elkrief L, Lannes A, Silvain C, Schnee M, Tanne F, Vavasseur F, Brusselle L, Brouard S, Kwok WW, Mosnier JF, Lohse AW, Poschmann J, Binder M, Gournay J, Conchon S, Milpied P, Renand A . Nat Commun. 2025 PMID: 39880819
Subclinical rejection-free diagnostic after kidney transplantation using blood gene expression. Danger R, Le Berre L, Cadoux M, Kerleau C, Papuchon E, Mai HL, Nguyen TV, Guérif P, Morelon E, Thaunat O, Legendre C, Anglicheau D, Lefaucheur C, Couzi L, Del Bello A, Kamar N, Le Quintrec M, Goutaudier V, Renaudin K, Giral M, Brouard S; DIVAT Consortium. Kidney Int. 2023 PMID: 36990211.
Effector Memory-Expressing CD45RA (TEMRA) CD8+ T Cells from Kidney Transplant Recipients Exhibit Enhanced Purinergic P2X4 Receptor-Dependent Proinflammatory and Migratory Responses. Doan Ngoc TM, Tilly G, Danger R, Bonizec O, Masset C, Guérif P, Bruneau S, Glemain A, Harb J, Cadoux M, Vivet A, Mai HL, Garcia A, Laplaud D, Liblau R, Giral M, Blandin S, Feyeux M, Dubreuil L, Pecqueur C, Cyr M, Ni W, Brouard S, Degauque N; on behalf on the DIVAT Consortium; DIVAT Consortium. J Am Soc Nephrol. 2022 PMID: 36280286
A fourth SARS-CoV-2 mRNA vaccine in strictly seronegative kidney transplant recipients. Masset C, Benotmane I, Dantal J, Garandeau C, Gauthier-Vargas G, Cantarovich D, Meurette A, Giral M, Caillard S, Blancho G. Kidney Int. 2022 PMID: 35167873
Urinary metabolomic profiling from spontaneous tolerant kidney transplanted recipients shows enrichment in tryptophan-derived metabolites. Colas L, Royer AL, Massias J, Raux A, Chesneau M, Kerleau C, Guerif P, Giral M, Guitton Y, Brouard S; DIVAT Consortium. EBioMedicine. 2022 PMID: 35241402
Updated on 25 February 2025.