Team 5 : Neuroinflammation, mechanisms, therapeutic options (NEMO)
Team 5 leaders: Pr. David Laplaud & Dr. Laureline Berthelot
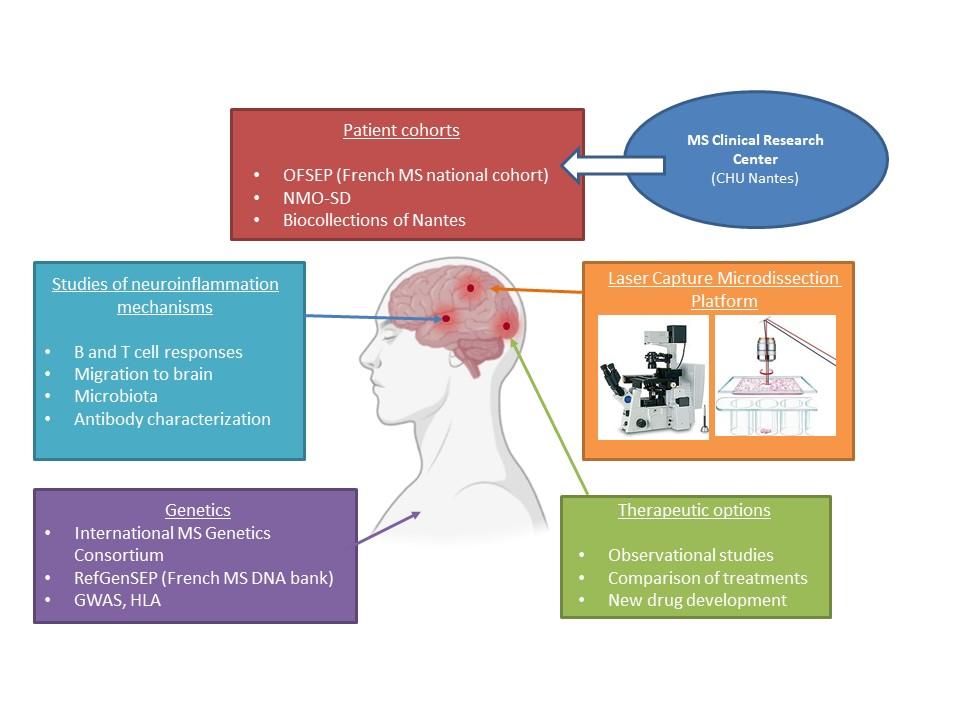
Team 5 focuses on the immune mechanisms underlying autoimmune demyelinating diseases of the central nervous system, studying the pathophysiology and treatment of multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). Our current research program aims at improving MS management, deciphering the immunopthology of MS linked to genetics. The developed research makes use of national biobanks (OFSEP) and collaborations with reputed national and international laboratories (IMSGC).
We particularly focus our fundamental research on CD8+ T cells and B cells in MS pathology, deciphering which subpopulations are involved and how they can be specifically targeted. In our research program, studies on antibody specificity linked to microbiota dysbiosis are developed. We also study the role of glial cell and antigen reactivity. Translational, genetic and clinical studies on MS and NMO complete the NEMO panel of research. We have access to and exhaustive biobank of MS patient samples (serum, saliva, LCS, stools, colon and brain sections, DNA collection: RefGenSEP). Animal models of MS (mice and rats) are also used to decipher pathological mechanisms and to test potential treatment as proof of concept.

Research program
Team 5 focuses on the immune mechanisms underlying autoimmune demyelinating diseases of the central nervous system, studying the pathophysiology and treatment of multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). Our current research program aims at improving MS management, deciphering the immunopthology of MS linked to genetics. The developed research makes use of national biobanks (OFSEP) and collaborations with reputed national and international laboratories (IMSGC).We particularly focus our fundamental research on CD8+ T cells and B cells in MS pathology, deciphering which subpopulations are involved and how they can be specifically targeted. In our research program, studies on antibody specificity linked to microbiota dysbiosis are developed. We also study the role of glial cell and antigen reactivity. Translational, genetic and clinical studies on MS and NMO complete the NEMO panel of research. We have access to and exhaustive biobank of MS patient samples (serum, saliva, LCS, stools, colon and brain sections, DNA collection: RefGenSEP). Animal models of MS (mice and rats) are also used to decipher pathological mechanisms and to test potential treatment as proof of concept.

Clinicians / Associate Researchers
Research assistants
Sonia BOURGUIBA-HACHEMI - IR
Axelle DURAND - IR
Julie EMERY - AI
Igor FADDEENKOV - IR
Alexandra GARCIA - IEH
Axelle DURAND - IR
Julie EMERY - AI
Igor FADDEENKOV - IR
Alexandra GARCIA - IEH
Postdoctoral fellows
Selected publications
 Durable B-Cell Impairment While Sparing IgA B Cells After Ocrelizumab Therapy in Multiple Sclerosis. Ann Clin Transl Neurol. 2025 Nov;12(11):2271-2285. PMID: 40781066
Durable B-Cell Impairment While Sparing IgA B Cells After Ocrelizumab Therapy in Multiple Sclerosis. Ann Clin Transl Neurol. 2025 Nov;12(11):2271-2285. PMID: 40781066Intrathecal Anti-Akkermansia muciniphila IgG Responses in Multiple Sclerosis Patients Linked to CSF Immune Cells and Disease Activity. J Clin Med. 2025 Aug 15;14(16):5771. PMID: 40869597
Investigating the metabolite signature of an altered oral microbiota as a discriminant factor for multiple sclerosis: a pilot study. Sci Rep. 2024 Apr 2;14(1):7786. PMID: 38565581
Acute clinical events identified as relapses with stable magnetic resonance imaging in multiple sclerosis. JAMA Neurol. 2024 Aug 1;81(8):814-823. PMID: 38949816
Highly effective therapies as first-in-line treatment for pediatric-onset multiple sclerosis. JAMA Neurol. 2024 Mar 1;81(3):273-282. PMID: 38345791
Aglycosylated extracellular loop of inwardly rectifying potassium channel 4.1 (KCNJ10) provides a target for autoimmune neuroinflammation. Brain Commun. 2023 Feb 22;5(2):fcad044. PMID: 36910419
Updated on 16 December 2025.