Cytometry and cell sorting
The technology
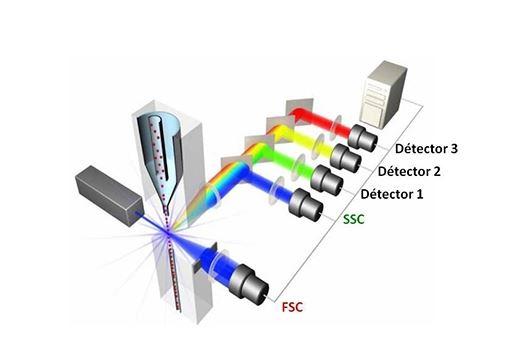
Flow cytometry allows the characterization of cells according to their size, their granulosity and the presence or absence of molecules of interest. Cells are labeled with fluorescent markers (eg: fluorescent antibodies, viability or apoptosis or proliferative markers,…) and pass in front of lasers. The markers excited by the lasers emit light at different wavelength. The emitted lights are collected and the fluorescence intensity is quantified for each fluorochrome to determine the amount and type of cells present in a sample.
Laws of Cytometry (or Shapiro's Laws)

Howard Shapiro is one of the founding fathers of Cytometry. He was the first in the 1970's to include several lasers in a cytometer. He determined some important rules in Cytometry.
Read them: they are full of common sense, still relevant and to respect!
Shapiro's Laws
Who is authorized to use cytometers and sorters?
Only people who have followed the mandatory training provided in the lab. All new users must follow the training.
Training provided on arrival of Master 2 students (on October and February) or on request for the other users, after a period of observation with the supervisor.
Our Instruments
- CYTEK AURORA Spectral Flow Cytometer
 With 64 fluorescent channels, Cytek® Aurora is able to detect the entire spectrum of the emitted light (between 365nm and 829nm). Each fluorochrome being characterized by its own spectral signature, this cytometer allows the simultaneously fluorochromes detection impossible to differentiated with the conventional cytometry (e.g. APC and Alexa Fluor 647, or GFP and FITC). It allows also the detection of new dyes without reconfiguring the system. The number of fluorescence’s in a same panel exceeds by far those measured in a conventional system. For example, it’s possible to use 33 different markers to characterize the different T cells sub-population on a single sample.
With 64 fluorescent channels, Cytek® Aurora is able to detect the entire spectrum of the emitted light (between 365nm and 829nm). Each fluorochrome being characterized by its own spectral signature, this cytometer allows the simultaneously fluorochromes detection impossible to differentiated with the conventional cytometry (e.g. APC and Alexa Fluor 647, or GFP and FITC). It allows also the detection of new dyes without reconfiguring the system. The number of fluorescence’s in a same panel exceeds by far those measured in a conventional system. For example, it’s possible to use 33 different markers to characterize the different T cells sub-population on a single sample.- 5 lasers : 355nm, 405nm, 488nm, 561nm and 640nm.
- SSC : 2 detectors with 405nm and 488nm bandpass filters.
- Volumetric sensor for the counts cells during the sample recording
- Plate loader
- FACSCelesta BD Biosciences

- 3 lasers: 405nm, 488nm, UV
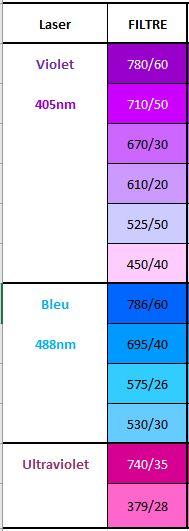
- 12 fluorescent detectors (FACS CELESTA Fluorochrome Table)
- BD High Throughput Sampler (HTS) for fully automated sample acquisition from microtiter plates
- Software: Digital data acquisition with software compensation
- FACSCANTO II BD Biosciences
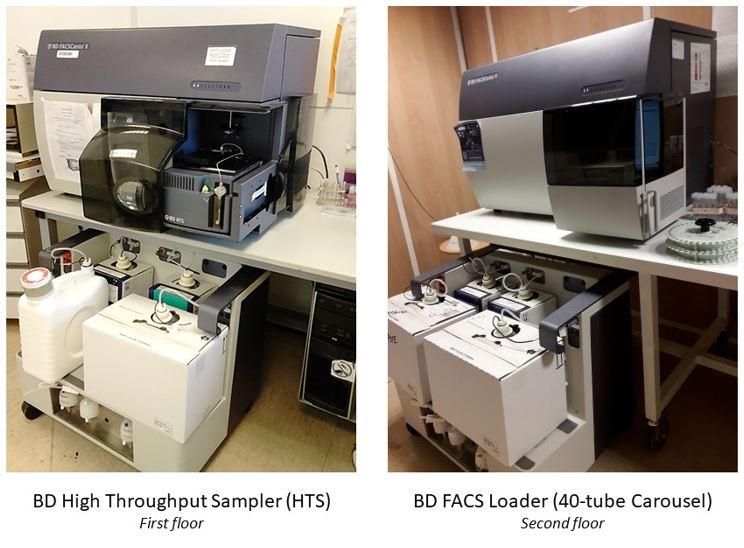
- 3 lasers: 405nm, 488nm, 633nm
- 8 fluorescent detectors (FACS CANTO Fluorochrome Table)
- Software: Digital data acquisition with software compensation, BD FACS DIVA
- FACSVerse BD Biosciences

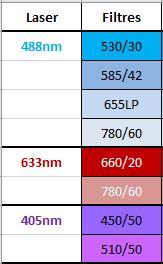
- 3 lasers: 405nm, 488nm, 633nm
- 8 fluorescent detectors (FACS Verse Fluorochrome Table)
- BD FACS Universal Loader for automated sample acquisition from tubes rack or from microtiter plates
- Software: Digital data acquisition with software compensation, BD FACSuite
For the use of this cytometer, contact:
- ARIA II BD Biosciences

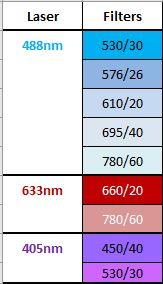
- 3 lasers: 405nm, 488nm, 633nm
- 8 fluorescent detectors (FACS ARIA Fluorochrome Table)
- Software: Digital data acquisition with software compensation, BD FACS DIVA
- Temperature control for sample and sorted populations
- Sort collection devices: Tubes (1.5mL, 5mL, 15mL), Plates (6/48/96 wells)
- AURORA Cell Sorter Cytek

- 3 lasers: 405, 488, 630nm
- Possible to use 24 dyes in combination in one panel
- Software: SpectroFlo (as Aurora analyzer)
- Temperature control for sample and sorted populations
- Sort collection devices: Tubes (1.5mL, 5mL, 15ml soon), Plates (6/48/96 wells)
- Sort up to 6 ways into 1.5ml tubes, or up 4 ways into 5ml tubes
Contact information
Updated on 23 June 2023.